 Source: A simple, comprehensive plan to prevent or reverse Alzheimer’s Disease and other neurodegenerative diseases – Part 1: The Plan | AGINGSCIENCES™ – Anti-Aging Firewalls™
Source: A simple, comprehensive plan to prevent or reverse Alzheimer’s Disease and other neurodegenerative diseases – Part 1: The Plan | AGINGSCIENCES™ – Anti-Aging Firewalls™
A simple, comprehensive plan to prevent or reverse Alzheimer’s Disease and other neurodegenerative diseases – Part 1: The Plan
By James P Watson, with contributions and editorial assistance by Vince Giuliano
INTRODUCTION AND OVERALL PRINCIPLES
This is the first of a pair of blog entries concerned with dementias – neurological diseases including Alzheimer’s Disease (AD) and its cousins. This Part 1 write-up was inspired by a recent small, non-randomized clinical trial done by Dr. Dale Bredesen that showed true “Reversal of Cognitive Decline” in 9 out of 10 patients with documented cognitive decline (Bredesen, 2014). Not all of these patients had AD, but all had cognitive decline. Five had AD, two had SCI (subjective cognitive impairment), and two had MCI (mild cognitive impairment). Although this study was too small to allow any statistical conclusions, it is the most positive report in a series of disappointing reports on the recent failures of Big Pharma’s monoclonal antibodies against amyloid-beta. Dale Bredesen’s approach was a multifactorial one – utilizing 24 different approaches to halt or reverse cognitive decline. We explore those 25 interventions here, focusing on the first 19. They do not depend on drugs. The focus of this blog entry is “What can be done about dementias now?”
The forthcoming Part 2 blog entry will provides a detailed discussion of some of the key science related to AD and dementias. This is the “What is science telling us about dementias?” part which gets quite complex. We review major theories related to AD there including the Hardy Hypothesis related to amloid beta, the GSK3 theory and more detail on the neuroinflammation theory which we introduce in this Part 1 blog entry. We expect to emphasize the emerging importance APP (Amloid Precursor Protein). And we will describe some very recent research that appears to establish that a basic cause of AD is the proliferation in aging of vestigal DNA segments in our genomes (known as LINEs which are long interspersed nuclear elements and SINEs which are short interspersed nuclear elements) with encode over and over again for the production of APP and for the failure of its clearance. This could well finally explain the role of beta amyloid in AD.
We have published a number of earlier blog entries relating to AD and dementias. For example, you might want to review my August 2014 blog entry The Amyloid Beta face of Alzheimer’s Disease.
About dementias
Dementia only happens to a minority of the population with aging, but is becoming an ever increasing problem with the explosion in longevity occurring world-wide
Cognitive decline is the major “fear” people have of getting old. Even individuals with the feared “ApoE4 polymorphism” are not “predestined” to develop Alzheimer’s Disease (AD). The ApoE4 allele is only a “risk factor” for AD, not the cause of AD.
A common error is that most people view “dementia” and “Alzheimer’s disease” as synonyms, but this is incorrect. Alzheimer’s disease is only responsible for 60% of cases of dementia in the US and even less of the cases in Japan. In the US, Vascular Dementia (VaD) is the second-most common cause of dementia (20%), whereas in Japan, the incidence of AD and VaD is almost the same. In the US, the remaining 20% of dementia cases are due to several other neurodegenerative diseases such as Lewy Body Dementia (LBD), Parkinson’s disease with dementia (PDwithD), Frontotemporal dementia/ALS spectrum disorder (FTD/ALS), and mixed dementia (which is usually a mixture of AD and VaD).
A portrayal of the breakdown follows.
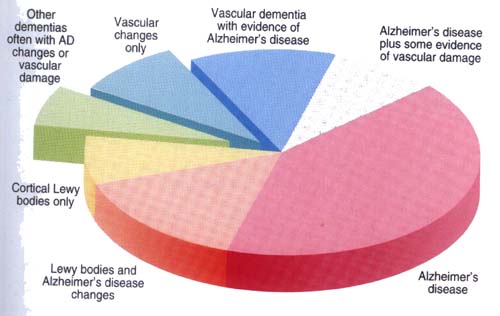
In the Middle East and China, VaD is more common than AD. This was true in Japan two decades ago, but now the ratio of AD to VaD is 1:1. Since AD and VaD are clearly the leading causes of dementia world-wide, we will focus mostly on these two types of dementia. Also, the risk factors for AD and VaD overlap and there are cases of “mixed dementia” which include features of both diseases. AD affects 5.4 million Americans and 30 million globally. By 2050, these numbers will be 13 million (US) and 160 million (world-wide) (Ferri, 2005). Many experts now regard dementia from neurodegenerative diseases as the 3rd leading cause of death after cardiovascular disease and cancer. Despite millions of dollars being spent annually on research, the exact causes of these dementias are still unknown, but the number of clues to the causes is growing and we will explore some of the main ones in our Part 2 blog entry.
Neuroinflammation is the most universally accepted explanation for AD
What is clear is that the “universal sign” of all neurodegenerative disease is “neuroinflammation”, which under the microscope is manifested as “gliosis” and is seen with AD, VaD, PD, FTD/ALS, and the type of dementia seen after multiple concussions, which is now called “Chronic Traumatic Encephalopathy” (CTE). Although they all have different “triggers” for each disease, they all have “neuroinflammation” and histologic signs of gliosis. We return to neuroinflammation several times as a central theme here and in the Part 2 blog entries.
Another “universal feature” is that all of these disease have familial cases with as few as 5% being genetic (AD) and as many as 50% being genetic (FTD). In these familial cases, there is most often a genetic mutation that is causal in nature (early onset disease) or a single nucleotide polymorphism (SNP) that is not causal in nature, but predisposes the patient to the disease. With the exception of CTE (where the primary cause is multiple concussions) and PD (where pesticide exposure, family history of PD, and depression combine to produce an odds ratio OR = 12.0), most of the cases of neurodegenerative dementias remain largely sporadic with unknown specific causation.
Environmental risk factors for neurodegenerative diseases are discussed in the 2005 publication Neurodegenerative Diseases: An Overview of Environmental Risk Factors and in publications in this list.
Despite millions of dollars being spent annually on research, the exact cause of these dementias are still unknown, but the number of clues to the cause is growing. What is clear is that the “universal sign” of these neurodegenerative diseases is “neuroinflammation”, which under the microscope is manifested as “gliosis” and is seen with AD, VaD, PD, FTD/ALS, and the type of dementia seen after multiple concussions, which is now called “Chronic Traumatic Encephalopathy” (CTE). Although they all have different “triggers” for each disease, they all have “neuroinflammation” and histologic signs of gliosis. Another “universal feature” is that all of these disease have familial cases with as few as 5% being genetic (AD) and as many as 50% being genetic (FTD). In these familial cases, there is most often a genetic mutation that is causal in nature (early onset disease) or a single nucleotide polymorphism (SNP) that is not causal in nature, but predisposes the patient to the disease.
With the exception of CTE (where the primary cause is multiple concussions) and PD (where pesticide exposure, family history of PD, and depression combine to produce an odds ratio OR = 12.0), most of the cases of neurodegenerative dementias remain largely sporadic with unknown specific causation.
Failure of Monotherapeutic Approaches to Neurodegeneration – It is time to consider multiple component therapies
The development of drugs to treat neurodegeneration has probably been the biggest failure of the pharmaceutical industry. Although there are three FDA-approved drugs for AD, none of them produce anything other than a marginal, unsustained effect on symptoms. Hundreds of clinical trials for AD have failed over the past two decades, most recently being the large Phase III trials of monoclonal antibodies that target amyloid-beta. As of today, no drugs have been approved for Frontotemporal dementia, Vascular dementia, and Lewy body dementia. Only one drug has been approved for Amyotrophic lateral sclerosis (ALS). All of the clinical trials done for these diseases have largely been with monotherapeutic drug approaches.
We know from the field of cardiovascular disease, cancer, and HIV that single drug therapy for these diseases largely fail. . It is now clear that cancer is “incurable” with chemotherapy unless multiple drugs are used. Combination therapies have become the standard for treating these conditions. The requirement to combine drug therapies appears to pertain as well to diseases that we cannot “cure” but that are are yet treatable: we can control the disease and prevent premature death from the disease. This includes cardiovascular disease, HIV, and a few other glaring chronic diseases. These diseases like dementias involve simultaneous upregulation or downregulation of hundreds or thousands of genes including protein-producing ones, and simultaneous activation or inhibition of a large multiplicity of pathway. It is a very tall order to find a single molecule that can have the right effects on so very many different upregulated and downregulated molecules and pathways at the same time. Yet, Big Pharma by tradition and because of patent law likes to look for single molecules that can be patented and that can make a big differences in a key step in a highly specific disease processes. But most serious aging-related diseases and dementias don’t offer such an opportunity.
The Multi-factorial approach rather than “single target” approaches to Treating Alzheimer’s Disease
For the same reasons, it makes sense that a single drug made by “Big Pharma” could NOT solve the problems with these neurodegenerative diseases. Here is a list of 25 different interventions that were combined into one effective program that “reversed” AD in 9 of 10 patients treated in a pilot study at UCLA and the Buck Institute. None of these involve drugs. I will include in black, the ones that were recommended by Dr. Dale Bredesen in what he calls the “MEND” program, which is an acronym that stands for “Metabolic Enhancement for NeuroDegeneration”. You can check out his 2014 paper Reversal of cognitive decline: A novel therapeutic program.
SECTION I PRACTICAL INTERVENTIONS
1. Eat a low glycemic, low inflammatory, low grain diet – Since sugar triggers insulin release and the insulin receptor triggers brain aging, this is easy to understand. For several complex reasons, certain proteins found only in grains (such as wheat germ, wheat gliadins) also triggers inflammation. The foods that have a high glycemic index or have lots of wheat in them include the following:
| High glycemic index foods (these are bad) (and pro-inflammatory nonglycemic foods) | Low glycemic index foods (these are good) (and anti-inflammatory foods and beverages) |
| Sweet Fruit – banannas, oranges, grapefuit | Fatty fruit – avocadoes, olives, capers |
| Orange juice, Apple juice, grape juice | Unsweetened coconut milk, soymilk, almond milk |
| Pancakes, waffles, French toast, toast | Scrambled eggs, omelettes, boiled eggs, fried eggs |
| Candy, Pies, Cake, Ice cream, Sherbert | Vegetables – Broccoli, Brussel sprouts, Artichokes |
| Corn bread, Cornflakes, corn oil | Olive oil, Coconut oil extract (MCT oil) |
| Processed cold cereals – Chex, Raisin bran | Oatmeal, barley cereal, rye bread, etc. |
| Cream of wheat, Fruit loops, etc. | Mushrooms, seaweed (Sushi), cheese, butter |
| Toast, bread, donuts, bagels, croissants | tomato soup (add some protein), mushroom soup |
| Potatoes, potato chips, French fries | Cream of broccoli soup, lentils, legumes |
| Sweetened yogurt, sweetened milk | Unsweetened yogurt, Greek yogurt |
| Cow’s milk, Chocolate milk, hot cocoa | Prosage patties, garden burgers, vegelinks |
| Jam, jelly, honey, maple syrup, pancake syrup | Soymeat, tofu, vegameat, Frichick |
| Peanut butter, Jam, and bread sandwiches | Portobello mushroom sandwiches w/o bread |
| White rice, brown rice, pita bread, wild rice | Indian curries (leave out the potatoes), Thai curry |
| Wheat thins, Pretzels, wheat snacks | Dried kale chips, seaweed snacks, onion snacks |
| Sugar drinks, sweetened tea, Gatoraid | Green tea, white tea (no caffeine), herbal teas |
2. Enhance autophagy – This can be done without fasting all day. Research has shown that fasting for at least 12 hours per day (evening and night) is sufficient to activate autophagy. Not eating for at least 3 hours before bedtime also activates autophagy. Eating the evening meal earlier in the day also helps. For those who do not want to fast for at least 12 hours, there may be little hope of “cleaning the cobwebs out of the brain”. Studies have shown that eating too much or eating late at night completely shuts off autophagy. This is probably the #1 reason why most people have so much “proteotoxicity” in the brain, the pancreas, and other organs. You can review our blog entry Autophagy – the housekeeper in every cell that fights aging.
There are some natural compounds and some drugs that stimulate autophagy, however. They include the following:
- mTOR inhibitors – The mTOR pathway is “downstream” from the Insulin/IGF-1 pathway. The mTOR pathway completely “shuts off” autophagy and stimulates protein synthesis. This is the primary “danger” of eating too much meat or protein (i.e. stimulating the mTOR pathway). Continually inhibiting the mTOR pathway is probably not a good idea either, since it is very important to synthesize proteins. However, intermittent mTOR pathway inhibition has been shown to be a very effective way of stimulating “cellular housekeeping” in the brain. The best-known drug that inhibits the mTOR pathway ia rapamycin. Low glucose levels and low amino acid levels in the blood also inhibit mTOR. It is interesting that at least one big pharma company, Novartis, is interested in marketing rapamycin as an anti-aging drug(ref).
- AMPK activators – The AMPK pathway is one of the major pathways that activates autophagy. AMPK is activated by both exercise and fasting. The AMPK pathway is a “cross-talk” pathway between mTOR and the Insulin/IGF-1 pathway. Activating AMPK inhibits both of these “bad” pathways. (They are only bad in certain contexts of aging and still serve important functions in aging people. We could not be alive without them. In the Part 2 blog entry we will talk about how some times IGF is the good guy we don’t want to be without.) Besides exercise and fasting, AMPK can be stimulated by three hormones, some drugs and many natural compounds. The most potent AMPK activator is muscle contraction (i.e. exercise). The three hormones that stimulate AMPK are thyroid hormone and two hormones secreted from fat: leptin and adiponectin. Next to this, the most potent chemical activators of AMPK are probably AICAR and ZMP. These are synthetic compounds that are the only true “exercise mimetics”. ZMP is a derivative of AICAR. AICAR has been shown to increase endurance in rodents by 44% without exercise. This is amazing. Combining AICAR with exercise makes the drug even more effective. Unfortunately, AICAR is very expensive ($350-450/gram). Common drugs that activate AMPK include metformin and aspirin. Natural compounds that activated AMPK include resveratrol, pterostilbene, curcumin, EGCG, betulinic acid, Gynostemma Pentaphyllum, Trans-Tiliroside (from rose hips), and 3-phosphoglycerate. See this list for articles in this blog that deal with autophagy or describe autophagy activators.
- Sirtuin activators – The 3rd major family of pathways that activates autophagy is for the Sirtuin enzymes (SIRT1-7). Sirtuins are enzymes that remove acetyl groups from proteins. The most important ones it deacetylates for autophagy are 3 proteins that are crucial to the autophagy system of “cellular housekeeping”. These 3 proteins are Atg5, Atg7, and Atg8. There are many practical reasons why activating Sirtuin-induced autophagy is critical to health. For instance, SIRT1 activation protects cells in human degenerative discs from death by promoting autophagy. This is why fasting has been shown to eliminate back pain. The most well-known SIRT1 activator is resveratrol, the active ingredient in red wine. However, both red wine and white wine have been shown to activate Sirtuin enzymes. NAD+, NMN, and NR all activate Sirtuin enzymes (all 7 of them), whereas resveratrol only activates SIRT1. You can see our blog entry NAD+ an emerging framework for health and life extension — Part 1: The NAD World
3. Reduce stress – psychological stress, depression, worrying, and being obsessive compulsive all increase the risk of Alzheimer’s disease. The most effective ways to reduce “cellular stress” are as follows:
- Yoga – yoga has been scientifically proven to reduce stress. The mechanism may be multifactorial, but studies suggest that activating stretch receptors in the muscles induces the SIRT3 gene. The Sirtuin pathway is a major pathway activated by fasting, caloric restriction, red wine, NAD+, NMN, NR, and certain other natural compounds.
- Meditation – meditation has been scientifically prove to reduce stress. However, 3 minutes of prayer is NOT meditation. Meditation requires 30-60 minutes of time. The MEND program recommends 20 minutes of meditation twice a day (No one prays that long).
- Tai chi – this ancient Chinese form of exercise has been shown to reduce stress
- Exercise followed by rest – exercise alone does not reduce stress, but exercise followed by a good night’s rest is very effective at reducing stress
- Stretching exercises – These have a special beneficial effect on stress, especially back stretching exercises for back pain.
Self-monitoring of daily stress and exercise can be helpful for knowing what your stress levels are and how good a job you are doing at keeping stress at non-harmful levels. A great many of the upstream conditions that can lead to dementias mentioned here (sedentary life style, improper diet, inadequate sleep, etc) are likely to induce constitutional stress which can be picked up by such monitoring. A host of new wearable devices can keep track of exercise and its consequences. See the blog entry Digital health – health and fitness wearables, apps and platforms – implications for assessing health and longevity interventions – Part 1. Vince has identified two constitutional stress measurements in his blog entry that can be tracked starting with smartwatch heart rate and sleep measurements, MRHR (morning resting heart rate before awakening), and ERHR-MRHR (difference between evening resting heart rate and morning resting heart rate during sleep, a measure of overnight sleep-related constitutional stress recovery),. These are described in the blog entry Digital health – health and fitness wearables, Part 2: looking for practical stress biomarkers. Also, heart rate variability is another personally trackable constitutional measurement of stress, See my recent blog entry on heart rate variability, Digital Health Part 3.
4. Optimize sleep – At least 8 hours of sleep at night is very effective in preventing Alzheimer’s disease.
Daytime sleeping probably is not as effective, but is probably not harmful provided that a person is not too sedentary with daytime sleeping (i.e. short naps). Adding 0.5 – 3 mg of melatonin and 500 mg of tryptophan is also very helpful in getting a good night’s sleep. One of the biggest problems with getting a good night’s sleep is sleep apnea, which is actually very common as we get older.
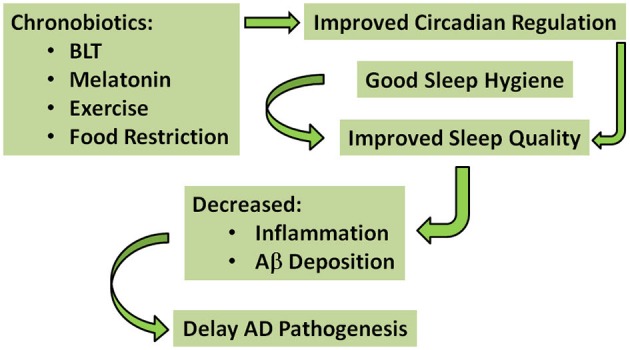
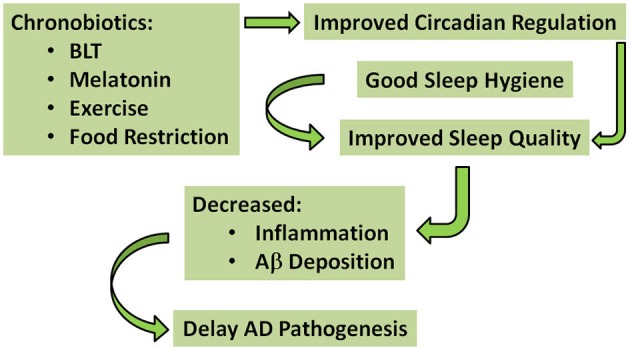
“A Simplified schematic of the proposed interventions that may have potential to delay AD pathogenesis — The green arrows indicate pathways for improved circadian regulation and sleep quality, ultimately delaying AD pathogenesis. According to this model, chronobiotics (i.e., bright light therapy (BLT); melatonin; exercise; and food restriction) and good sleep hygiene could be used individually—but preferably in combination—to improve circadian regulation and sleep quality, decrease inflammation and Aβ deposition, and thereby delay AD pathogenesis.” Image and legend source
5. Exercise – The World Health Organization recommends 150 minutes of exercise per week, but the best scientific evidence suggests that this is NOT enough. The best scientific evidence suggests at least 450 minutes of exercise per week. That is 60 minutes per day and an extra 20 minutes on one of those days. If you want to skip Saturday, that means 75 minutes per day (1hr 15 minutes). The exercise should include the following for preventing Alzheimer’s disease:
- Swimming, outdoor hiking, calisthenics, aerobic fitness classes, spinning classes, etc.
- 30-45 minutes of aerobic exercise where the heart rate is 60% of training heart rate. This can be on a stationary bicycle, an elipical machine, a “hand bicycle”, a stair climber,
- 1 mile per day of walking outside (the speed is not important)
- Resistance exercise – this includes weight lifting, machines, stretch bands, push-ups, etc.
- Stretching – stretching activates stretch receptors which activates the SIRT3 gene, which is key for mitochondrial function and decreasing free radicals in the muscles (which cause pain
- Listening to relaxing music – classical music listening is a good way to relax.
Watching TV or looking at a computer screen and “surfing on the computer” probably does NOT work to reduce cellular stress. Here are some of the blog entries we have published relating to exercise.
6. Brain stimulation – The Mayo Clinic did a study in 487 patients where they participated in a computerized cognitive training program called “Brain Fitness Program” by Posit Science. This computer training required 1 hour of time per day, 5 days per week for 8 weeks (totaling 40 hours). This was called the IMPACT study. This program increased their auditory processing speed by 131% and improved their memory an equivalent of approximately 10 years! Here is some information on this inexpensive computer program:
- Posit Science Brain Fitness Program for One Person
- PositScience Brain Fitness Program Classic (PC Version)
Some of us think that we may keep our brains fit by constantly trying to figure out the mechanisms of aging.
7. Keep your homocysteine low – High homocysteine levels seem to correlate with inflammation and also with deficiencies in folate cycle intermediates. The MEND program recommendation is to check your homocysteine levels and if it is > 7, then to take methyl-B12, methyltetrahydrofolate, pyridoxal-5-phosphate, and trimethylglycine (if necessary). The dosages are: Methyltetrahydrofolate – 0.8 mg/day and Pyridoxine-5-phosphate – 50 mg/day
8. Keep your vitamin B12 high – Vitamin B12 is very important in memory and prevention of dementia. Vit B12 deficiency alone can cause dementia. It is easier to prevent than to reverse. The MEND program recommends taking methyl-B12, not regular B12. They recommend basing the dose of methyl-B12 on serum levels of B12, which they recommend keeping above 500 with 1mg of methylB12/day.
9. Keep your C-reactive protein low – CRP is a measure of inflammation. This correlates very well with inflammation in the brain (called neuroinflammation). They recommend keeping the CRP levels below 1.0 and the Albumin/globulin ratio > 1.5. There are no FDA-approved drugs that lower this which are safe to be used on a chronic basis. However, there are several natural products that are effective in reducing C-reactive protein (CRP). They include curcumin (400 mg/day), Fish oil (DHA & EPA), and an anti-inflammatory diet that is low in sugar and wheat products. The MEND program recommends 700 mg of DHA twice a day (total 1400 mg) and 500 mg of EPA twice a day (total 1,000 mg). Since most Fish oil capsules are only about 1/3rd omega-3 fatty acids, that means you need to take about 7,000-8,000 mg (i.e. 7-8 one gram capsules) per day of Fish oil.
10. Keep your fasting insulin low – Most people develop insulin resistance with aging. Unfortunately, this is rarely diagnosed until they have already suffered the consequences of insulin resistance, which include metabolic syndrome, hypertriglyceridemia, hypercholesterolemia, Alzheimer’s disease osteoarthritis, accelerated hearing loss, accelerated visual impairment (including presbyopia, cataracts, and age-related macular degeneration, aka AMD). Once these things occur, then reducing your fasting insulin no longer is useful – the cells are already dead! The MEND program recommends keeping your fasting insulin to < 7.0. The best way to do this is to eat a low glycemic index diet, encourage ketogenesis by 12 hours of fasting per day, exercise, sleep, and in some cases the drug metformin. We have found that the NAD precursor, NMN is effective in reducing fasting insulin levels. Other supplements designed to enhance NAD+ may help as well.
11. Hormone balancing – The MEND program recommends normalizing thyroid hormone levels (free T3, free T4, estrogen, testosterone, progesterone, pregnenolone, and cortisol). For most people, cortisol levels are way too high. The best way to reduce cortisol is to reduce stress, improve sleep, and also possibly to supplement with NMN or NR. The rest of the hormones decline with aging and often need replacement. Here are some ways to make this safe:
- Testosterone replacement therapy – this is risky in older men, due to the risks of accelerated coronary artery narrowing due to neointimal hyperplasia, as well as benign prostatic hypertrophy worsening or by making prostate cancer grow. For this reason, a thorough work-up for prostate cancer must be done before starting testosterone. In addition, testosterone dosing should be based on testosterone levels.
- Progesterone – This is primarily for women, but also helps men in low doses. Any progesterone replacement therapy should also be based on blood levels of progesterone.
- Pregnenolone – This helps both men and women for the brain.
- Estradiol (E2) – This should also be done based on blood levels of E2
12. Healthy gut bacteria – Most people have very unhealthy gut bacteria due to the use of antibiotics, due to general anesthesia, and due to dietary factors such as a high sugar diet. As a result, the lactobacillus that are good for your health often die. In addition, the fiber-fermenting bacteria are often absent, thereby eliminating the healthful effects of a high fiber diet. Probiotics and prebiotics are often helpful in restoring healthy gut bacteria. You can see Vince’s 2012 blog entry Gut microbiota, probiotics, prebiotics and synbiotics – keys to health and longevity.
13. Reducing amyloid beta aggregates – One of the hallmarks of Alzheimer’s disease is the accumulation of misfolded, aggregates of a protein called amyloid beta. Fortunately, there are two natural compounds that if taken in large quantities can reduce amyloid-beta plaques in the brain. They are Ashwagandha and curcumin. Both of these are effective in reducing amyloid beta plaques. The MEND program recommend doses of 500 mg for Ashwagandha and 400 mg for curcumin. Because curcumin is so poorly absorbed, it is better to take a liposomal or nanoparticle form of the curcumin, like Bio-curcumin 95. Curcumin can be taking as a pill, but it may be absorbed much better in curry that has coconut oil, since the coconut oil creates an emultion and micelles which can be absorbed by the lymphatic system and thereby “bypass” the liver and the “first pass effect”. Ashwagandha is much better absorbed and does not have as much of a problem. It can be taken as a pill, but also can be taken as a tea. My friend Dr. Vince Giuliano has made a liposomal form of these two compounds together with two complementary anti-inflammatory herbal extracts which he believes get into the blood stream in concentrations that are 8-10 times higher than by pill form. He has written about these and other phytosubstances a number of times, e.g.(ref) (ref) (ref) (ref) (ref).
14. Cognitive enhancement – This category was probably added to the MEND program for supplements that could not be categorized elsewhere. They specifically recommend the natural product called Bacopa monniera and Magnesium. Bacopa monnieri is also called “water hyssop”, “herb of grace”, “Indian pennywort” and Withania somnifera. Bacopa monniera has been shown to reduce amyloid plaque and prevent synaptic decline in mouse models of AD. One possible mechanism by which Bacopa monnieri works is to enhance LDL receptor-related protein, which is the “amyloid exporter” in the brain. There are many studies that show a benefit from Bacopa monniera In humans. A meta-analysis of 6 high quality clinical trials of Bacopa monniera showed that 9 out of 17 tests showed improved performance in the domain of “memory free recall”. In a study on Okadaic acid induced memory impaired rats, the effect of standardized extract of Bacopa monnieri and Melatonin on the Nrf2 pathway was investigated. “OKA caused a significant memory deficit with oxidative stress, neuroinflammation, and neuronal loss which was concomitant with attenuated expression of Nrf2, HO1, and GCLC. Treatment with BM and Melatonin significantly improved memory dysfunction in OKA rats as shown by decreased latency time and path length. The treatments also restored Nrf2, HO1, and GCLC expressions and decreased oxidative stress, neuroinflammation, and neuronal loss. Thus strengthening the endogenous defense through Nrf2 modulation plays a key role in the protective effect of BM and Melatonin in OKA induced memory impairment in rats.” There is a special form of magnesium which is much better incorporated into the cell called Magnesium-L-threonate, aka MgT. Both can be taken as a capsule. The dose Bacopa monniera they recommend is 250 mg/day. However, most of the clinical trials recommend dosages of 300-450 mg/day.
15. Vitamin D3 –Vitamin D3 seems to be quite different than the other vitamins for a variety of reasons. The most important difference is that Vitamin D levels should be checked and individuals need to adjust their dose based on their serum vitamin D3 levels. To prevent AD, the levels of Vitamin D3 need to be > 50 nmol/L. The strongest evidence for this comes from two recent studies from 2014. One was a 5 year study in 1,658 elderly patients who started the study with no dementia. During the 5 years, 171 of the 1,658 developed dementia (10% risk over 5 years). This study looked at “all cause dementia”, of which 90% is Alzheimer’s dementia (AD) and Vascular dementia (VD). The risk of developing dementia when serum Vitamin D3 levels were > 50 nmol/L was very low. However, those with Vit D3 levels between 25 and 50 nmol/L had a 1.53 fold higher risk of developing dementia of any type. Those with levels below 25 nmol/L had a 2.25 nmol higher risk of developing dementia of any type. The 2nd study reported in 2014 was from Denmark and followed 10,186 individuals in the Danish population for 30 years. They looked at the risk of specific kinds of dementia and the relationship to Vitamin D3. For Alzheimer’s disease (AD), the risk of AD type dementia was 1.25-1.29 fold higher in those with serum Vit D3 levels below 25 nmol/L. For Vascular Dementia (VD), the risk of VD type dementia was 1.22 fold higher in those with serum Vit D3 levels below 25 nmol/L. In conclusion, low Vitamin D3 levels is one of the largest risk factors for dementia and the easiest to prevent. Most people do not get their Vitamin D3 levels checked. Do you know what yours is?
16. Increasing Nerve Growth Factor (NGF) – Hericium erinaceus and ALCAR — Although there are many growth factors that make nerve cells grow, the most important one is probably Nerve Growth Factor (NGF). NGF is a growth factor made and secreted by astrocytes in the brain and spinal cord. NGF enhances neuronal stem cell regeneration of the brain. Exercise is a potent stimulator of NGF secretion. There are several natural compounds that stimulate nerve growth factor secretion. They include extracts from the mushroom, Hericium erinaceus. Although there are other edible mushrooms that are good for you, of the 4 edible mushrooms that were studied for their effect on NGF secretion, only Hericium erinaceus induced the secretion of NGF from human astrocytes in the Hippocampus of the brain. Another compound that stimulates the secretion of NGF is Acetyl-L-carnitine, aka ALCAR. Acetyl-L-carnitine also helps with neuropathic pain. In rodent models of Alzheimer’s disease, 150 mg/kg/day of ALCAR induced NGF secretion and increased choline acetyltransferase activity, which increasea acetylcholine levels in the hippocampus.
17. Provide the substrates for synaptic formation – uridine, choline, citocolin, DHA, EPA, and herring roe — The ability to form synaptic connections between neurons is a key part of forming memory. Several key molecules are needed to create these synapses and dendritic spines that are not made by the human body (e.g. DHA) or are made in inadequate amounts (e.g. citicoline). The omega-3 fatty acid called docosahexaenoic acid (DHA) is probably the “rate-limiting substrate” in the formation of presynaptic and postsynaptic proteins. DHA alone will increase the formation of synapses and increase cognitive performance in humans and experimental animals, but the addition of two other circulating precursors for phosphatidylcholine also enhance memory formation. These two other precursors are uridine (which gives rise to brain UTP and CTP) and choline (which gives rise to phosphocholine). Phosphatidylcholine (PC) is the major phosphatide found in human neuronal connections. The other two major synaptic ingredients are uridine and DHA. Studies have shown that the aministration of choline, uridine, and DHA together have a greater effect than the sum of the individual effects (i.e. they have a synergistic effect on generating synapses and dendritic spines). DHA alone increased the synthesis of hippocampal phospholipids by 8-75%, with the greatest percentage being in the synthesis if PC (phosphatidylcholine). There are still controversies as to how much DHA a person should take per day.
The MEND program recommends 320 mg of DHA/day, but other experts recommend as much as 2,000 mg/day of DHA. Herring roe, the eggs from the Herring forage fish, is another good source of n-3 polyunsaturated fatty acids that have a high phospholipid content. MOPL 30 is a supplement product made by Artic Nutrition that includes a lot of phospholipids and a 3:1 ratio of DHA:EPA. The MOPL 30 proprietary supplement not only increased neuronal generation, it also decreased plasma triacylglycerol and non-esterified fatty acids as well as increased HDL-cholesterol. Although fasting glucose did not change, the glucose measurement on OGTT decreased at 10 minutes and 120 minutes into the test. Instead of taking herring roe, uridine, or choline, the MEND program recommends citocoline (aka CDP-Choline) an intermediate compound in the generation of phosphatidylcholine from choline (i.e. already half made). It is marketed under many names worldwide, including Ceraxon, Cognizin, NeurAxon, Somazina, Synapsine, etc. Studies have shown that citocoline increases dopamine receptor densities, prevents memory impairment, improve focus and mental energy. Citocoline may also help treat attention deficit disorder (ADD). The MEND program recommends a dose of 500 mg of Citocoline twice a day, 320 mg of DHA per day, and 180 mg of EPA per day.
18. Optimize antioxidants – mixed tocopherols, tocotrienols, Selium, blueberries, NAC, Vit C, a-lipoic acid. Although the free radical theory of aging has largely been proven to be incorrect as the “cause of aging”, there is no question that the “effect of aging” includes free radical damage to proteins, lipids, and nucleic acids that make up a cell. To try to mitigate these “downstream effects” of aging, many believe that the judicious use of antioxidants still plays a useful role in treating neurodegeneration. In this blog we have questioned that viewpoint and have pointed out that “antioxidants” like those mentioned often have powerful epigenetic impacts that better explain their actions(ref)(ref).
19. Optimize Zn:fCu ratio – Alzheimer’s disease may be caused (in part) by copper toxicity — The fact that Alzheimer’s disease was rare prior to 1900, yet now being very common has led many experts to look for environmental “causes” of AD. One of the leading “suspects” in a long list of environmental risks for AD is inorganic copper, which comes from drinking water and supplement pills. There is clear evidence from human subjects that serum free copper is elevated with AD and that the level of free copper in the serum correlates with cognition and predicts cognition loss. Animal studies have replicated these findings and have shown that as little as 0.12 ppm of coper in distilled drinking water in cholesterol-fed rabbits greatly enhanced the formation of AD.
A 2nd feature of AD is that those affected also have Zinc deficiency. A small clinical trial published in 2014 showed that in patients over the age of 70, Zinc supplementation protected against cognitive loss and also reduced serum free copper levels in AD patients. For these reasons, it is unclear if the efficacy of Zinc therapy is on restoring normal Zn levels or if it is due to reducing Cu levels.
The following Table lists the remaining interventions in Dale Bredesen’s list. These are fairly clear and we will not expand on them here.
| 20. Ensure nocturnal oxygenation | Exclude or treat sleep apnea | [54] |
| 21. Optimize mitochondrial function | CoQ or ubiquinol, α-lipoic acid, PQQ, NAC, ALCAR, Se, Zn, resveratrol, ascorbate, thiamine | [55] |
| 22. Increase focus | Pantothenic acid | Acetylcholine synthesis requirement |
| 23. Increase SirT1 function | Resveratrol | [32] |
| 24. Exclude heavy metal toxicity | Evaluate Hg, Pb, Cd; chelate if indicated | CNS effects of heavy metals |
| 25. MCT effects | Coconut oil or Axona | [56] |
Neuroinflammation “causes” all of the neurodegeneratove diseases
Although we will save most of our discussion on the science of AD to the coming Part 2 blog entry in this series, we comment here a bit more on the the science behind most of the above interventions – their neuroinflammatory nature.
In all neurodegeneratiave diseases (both familial and sporadic cases), there is evidence of a chronic, low grade brain inflammation that does not go away. Histologically, this is called “gliosis”, a term that describes what is seen under the microscope. As mentioned above, microglial cells are increased in number and they appear “angry” (i.e. they are activated) likely due to the presence of Aβ1-42. It is likely that these microglial cells are secreting pro-inflammatory factors which are causing the inflammation, although the picture is actually much more complex. Vince has written about this in 2011 and before in the blog entries Key roles of glia and microglia in age-related neurodegenerative diseases, New views of Alzheimer’s disease and new approaches to treating it, and Alzheimer’s Disease Update. We surface some additional insights here and in Part 2..
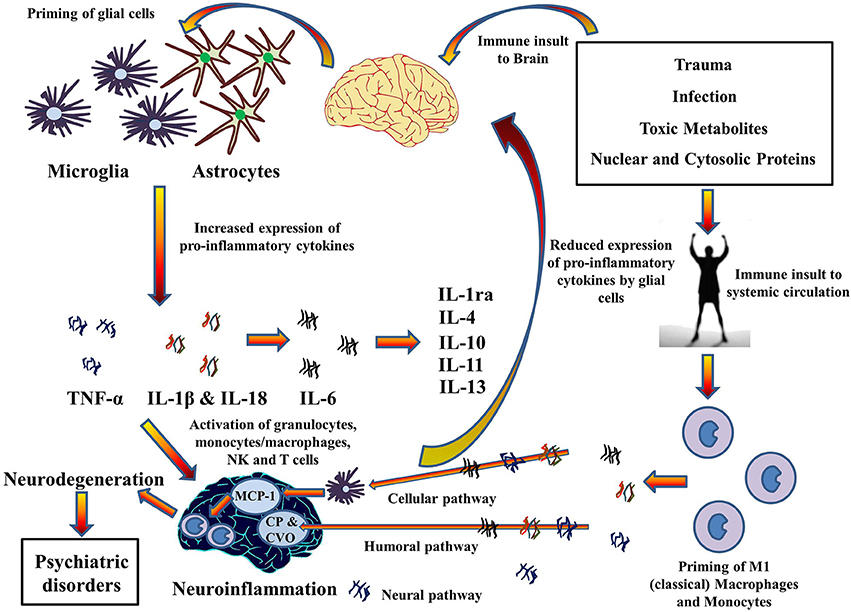
This illustration portrays some of the inflammatory processes that go on when microglia and astrocytes are activated:
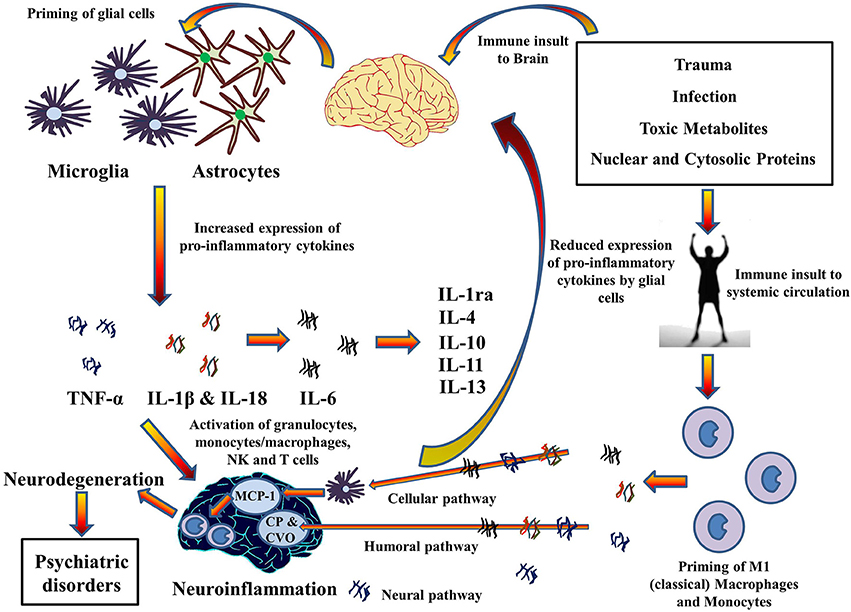
Image and legend source The 2014 publication Inflammasomes in neuroinflammation and changes in brain function: a focused review “Cytokines hypothesis of neuroinflammation: Implications in comorbidity of systemic illnesses with psychiatric disorders. Pro-inflammatory cytokines can migrate between systemic circulation and brain in both directions which could explain the comorbidity of systemic illnesses with psychiatric disorders. There are three pathways for the transport of pro-inflammatory cytokines from systemic circulation to brain as described by Capuron and Miller (2011): Cellular, Humoral, and Neural. Moreover, PAMPs and DAMPs from trauma, infection, and metabolic waste can prime glial cells to express pro-inflammatory cytokines TNF-α, IL-1β, and IL-6. When expressed, these cytokines activates granulocytes, monocytes/macrophages, Natural Killer, and T cells and together contribute to the pathophysiology of neuroinflammation. Chronic neuroinflammation could result in neurodegeneration and associated psychiatric disorders. These pro-inflammatory cytokines also stimulate production and expression of anti-inflammatory cytokine by glial cells that function as negative feedback to reduce the expression of pro-inflammatory cytokines, subsiding the neuroinflammation. MCP-1, Monocyte chemoattractant protein-1; CP, Choroid plexus; CVO, Circumventricular organ.”
The chronic inflammation viewpoint of Alzheimer’s disease is related to but somewhat different than the Beta Amloid viewpoint, the viewpoint covered in my recent blog entry The Amyloid Beta face of Alzheimer’s Disease.
The situation is described in a 2014 publication by Landry and Liu-Ambrose: “An alternative to the classic amyloid centric view of AD suggests that late-onset AD results from age-related alterations in innate immunity and chronic systemic inflammation (for review see Krstic and Knuesel, 2013).
In the Part 2 blog entry we will go into the neuroinflammation hypothesis in further depth and will explore other theories as to causes of AD and the other neurodegenerative diseases.
So, a basic strategy for preventing or delaying the onset of neurodegenerative diseases is to mount a multifront war on systematic inflammation. The 25 Bredesen interventions described above are initiatives in that war.