Metformin a life extension drug?
 by Sven Bulterijs
by Sven Bulterijs
For hundreds of years it has been known that a plant extract from the French lilac (Galega officinalis) improved the intense urination seen in diabetes patients. From this plant guanidine was extracted but it was too toxic for human use. In the 1920s another extract – galegine – of the French liliac was briefly used followed by synthetic analogs – Synthalin A and B. In the 1950s artificial drugs – phenformin, butformin and metformin – with a chemical similarity to guanidine became available for clinical use. In the 1970s however phenformin and buformin were withdrawn from the market because of the increased risk for lactic acidosis (1,2), a severe disease with a mortality rate of about 50% (3). A safer analog of these drugs, metformin, is now probably the most widely prescribed antidiabetic drug in the US (1). It is also used to treat polycystic ovary syndrome (PCOS), an endocrine disorder, leading to anovulation (failure to release the egg cell from the ovaries) (4).
Calorie restriction (CR) is the best investigated experimental technique to extend lifespan of a wide range of organisms from yeast to rats (5). Metformin has been suspected for quite a while to be a CR mimetic – a drug that mimics the effects of a CR diet. Indeed, Stephen Spindler found that short-term (8 weeks) of metformin supplementation mimicked 75% of the gene expression changes observed in long-term CR. In contrast, short-term (8 weeks) CR only mimicked 71% of gene expression changes (6). Thus the metabolic effects of metformin supplementation and CR are very similar and suggest that metformin will, like CR, extend the lifespan and reduce the incidence of many degenerative diseases.
Buformin, phenformin, and metformin have been shown to extend the mean and maximal lifespan in mice, rats, and C. elegans – a tiny round worm and one of the most popular model organisms in biological research (4,7,8). It also reduces spontaneous and carcinogen-induced tumor incidence in normal and cancer susceptible mice, rats and hamsters (9,10). Recently it was found that injection of metformin decreased tumor burden by a remarkable 72%, tumor volume by 50%, and multiplicity by 66% in a toxin-induced mice model for lung cancer (11). Epidemiological studies have shown that diabetes patients treated with metformin have a lower risk for cancer compared to those treated with other anti-diabetic drugs (12,13). The use of metformin as an adjuvant therapy in the treatment of cancer is currently under investigation. Finally, metformin also has a positive effect on a wide range of risk factors for coronary heart disease (CVD) such as decreases in plasma triglycerides, total cholesterol, LDL, Lp(a), free fatty acids, and CRP while slightly increasing HDL (4). However, a recent meta-analysis found no significant harm nor benefits of metformin therapy on cardiovascular events (14).
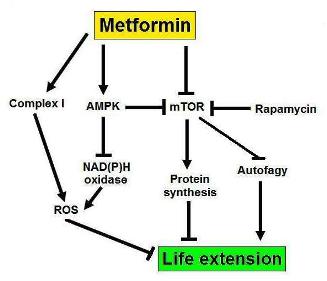 Fig. 1 Pathways influenced by metformin that explain its life extension properties. The blunt ending arrows indicate inhibition while the arrows indicate activation.
Fig. 1 Pathways influenced by metformin that explain its life extension properties. The blunt ending arrows indicate inhibition while the arrows indicate activation.
The mechanisms for lifespan extension by metformin are not completely understood yet but probably involve AMPK and its downstream target mTOR (fig. 1) (4). mTOR is also the target of the famous life extension drug rapamycin that increases lifespan in mice both when administered early and during middle life (15,16). mTOR inhibits autophagy thus metformin activates autophagy by inhibiting mTOR. Autophagy clears cells from dysfunctional organelles – such as mitochondria – and other junk that can impair normal cellular functions and thereby extends lifespan. mTOR stimulates protein synthesis and for still very incomplete understood reasons protein synthesis reduces lifespan. Therefore again metformin is expected to retard aging. If the cell cycle is arrested – that is the cell temporally stops dividing – and the cell continues to receive growth signals (mTOR stays active), it can lead to cell senescence – a state of permanent cell cycle arrest (4). Senescent cells secrete proinflammatory molecules, this has been termed the senescence-associated secretory phenotype, and can locally stimulate tumor growth (17). Metformin is expected to prevent cell senescence by removing the growth signal pressure. AMPK inhibits NAD(P)H oxidase, an enzyme whose action produces superoxide – a free radical. Metformin thus decreases free radical production (4). Free radicals are molecules that have an unpaired electron, they desperately try to find an extra electron to stabilize their configuration and in the process destroy other molecules by stealing their electrons. The free radical theory of aging is without doubt the most well known mechanistic aging theory. It was first proposed by Denham Harman in 1956 (18). Since then the failure of antioxidants to extend the lifespan has cast doubt on the validity of the free radical theory but still most biogerontologists think that free radicals do play a certain role. In fact some even suggest that the failure of antioxidants is expected because they undermine the bodies stress response (19). AMPK can be activated by: (i) binding of AMP, and (ii) phosphorylation by other enzymes. Metformin has clearly been shown to increase the phosphorylation of AMPK but the effects on AMP are disputed. Phenformin and buformin inhibits complex I of the respiratory chain, so maybe metformin does so too. Such an inhibition would decrease ATP – the universal energy currency for life – production and thereby increase AMP levels.
ADP + Pi ⇔ ATP
ADP + ADP ⇔ AMP + ATP
AMP acts as an activator for AMP and thus complex I inhibition would activate AMPK. This one year study found that metformin inhibits complex I (20) while another one found no such inhibition (21). This is good and bad news. The inhibition of complex I is responsible for lactic acidosis induced by phenformin and buformin and since metformin does not inhibit complex I it is much safer. Indeed, in a meta-analysis of published reports and controlled trials for a total of 36000 patient years of metformin exposure, Salpeter et al. found no cases of metformin-induced lactic acidosis (22). However, several case reports have been published in the literature (23). A common factor among all cases is that the patients have contra-indicators for the use of metformin such as renal problems and heart failure. Of the 47 patients with confirmed metformin-induced lactic acidosis by 1998 only 4 did not have any contra-indications for its use and all 4 recovered (24). The bad news is that complex I inhibition is expected to decrease free radical leakage (4). Metformin has been shown to prevent protein cross-linking (4).
Chemically metformin is very similar to aminoguanidine (fig. 2), one of the most researched inhibitors of glycation (for a review on glycation and protein cross-linking see 25). AGEs activate RAGE – a cell membrane receptor – leading to inflammation. Metformin has been shown to reduce inflammation in cells exposed to AGEs (26). Metformin reduces IGF-1 – a peptide hormone that stimulates growth. Mice with mutations in the growth hormone signaling pathway have lover IGF-1 levels and life 25-60% longer than control mice (27,28). Based on research in C. elegans and some suggestive evidence in mice (lower IGF-1 in the growth hormone pathway mutant mice) it has been concluded that decreasing IGF-1 will probably extend lifespan (29). Recently it was shown that metformin protects against replicative stress (30).
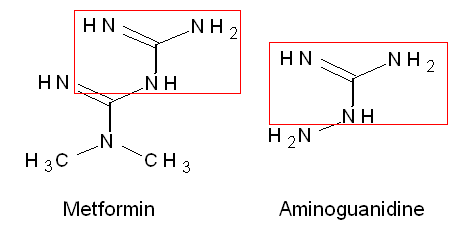 Fig. 2 Structural relationship between metformin and aminoguanidine. Both contain a guaninium-group (as is also found in the side chain of arginine).
Fig. 2 Structural relationship between metformin and aminoguanidine. Both contain a guaninium-group (as is also found in the side chain of arginine).
In my recent review (4) I have suggested that AMPK might not be involved in life span extension by CR because of the inconsistent results of several studies on how CR influences AMPK (activation, no-activation, or inhibition). AMPK might however ’intersect’ with the ‘CR-pathway’ and thus I’ve termed metformin an ‘indirect CR mimetic’ (fig. 3). This model also explains why CR and metformin have some different metabolic effects.
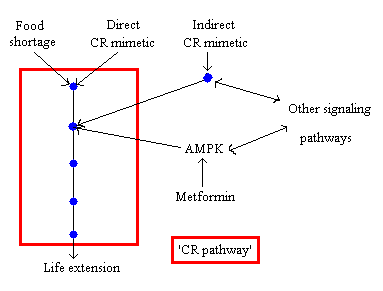 Fig. 3 Metformin as an indirect CR mimetic. The blue dots represent different steps (protein cascade) in the ‘CR pathway’. Note that even though for simplicity the pathway is represented as a linear series of steps, in reality it will be a complex network that intersects with many other pathways. The double arrow between AMPK and ‘other signaling pathways’ illustrates that other signaling pathways can activate AMPK and thus influence the ‘CR pathway’ but that activation of AMPK, for example by metformin, can also activate other pathways that are independent of the ‘CR pathway’ (idem dito for the other double arrow) (see: ref. 4).
Fig. 3 Metformin as an indirect CR mimetic. The blue dots represent different steps (protein cascade) in the ‘CR pathway’. Note that even though for simplicity the pathway is represented as a linear series of steps, in reality it will be a complex network that intersects with many other pathways. The double arrow between AMPK and ‘other signaling pathways’ illustrates that other signaling pathways can activate AMPK and thus influence the ‘CR pathway’ but that activation of AMPK, for example by metformin, can also activate other pathways that are independent of the ‘CR pathway’ (idem dito for the other double arrow) (see: ref. 4).
Rapamycin is a very expensive drug, about 500 USD a month for a daily dose of 2mg. Metformin, on the other hand, is extremely cheap and its side effects are more moderate. Metformin does inhibit vitamin B12 absorption in about 10% (but in some studies up to 30%) of treated patients (31,32). Thus blood levels vitamin B12 should be regularly tested. In conclusion we can say that metformin is an interesting candidate for a cheap and safe CR mimetic.
NOTE: Statements herein have not been evaluated by the FDA or any regulatory body. LongeCity does not promote the off-label use of any drug without appropriate medical supervision.
References:
- (1) Hundal RS, Inzucchi SE. Metformin. New understandings, new uses. Drugs 2003;63:1879–1894.
- (2) Bailey CJ, Day C. Metformin: its botanical background. Practical Diabetes Int 2004;21(3):115-117.
- (3) Silvestre J, Carvalho S, Mendes V, Coelho L, Tapadinhas C, Ferreira P, Povoa P, Ceia F. Metformin-induced lactic acidosis: a case series. J Med Case Rep 2007;1:126.
- (4) Bulterijs S. Metformin as a geroprotector. Rejuvenation Res 2011;14(5):469-482.
- (5) http://www.longecity…tion-primer-r25
- (6) Spindler SR. Use of microarray biomarkers to identify longevity therapeutics. Aging Cell 2006;5:39–50.
- (7) Anisimov VN, Semenchenko AV, Yashin AI. Insulin and longevity: Antidiabetic biguanides as geroprotectors. Biogerontology 2003;4:297–307.
- (8) Anisimov VN. Metformin for aging and cancer prevention. Aging 2010;11:1–15.
- (9) Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zaberzhinski MA, Poroshina TE, Semenchenko AV, Provinciali M, Re F, Franceschi C. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol 2005;40:685–693.
- (10) Anisimov VN, Egormin PA, Bershtein LM, Zabezhinskii MA, Piskunova TS, Popovich IG, Semenchenko AV. Metformin decelerates aging and development of mammary tumors in HER-2/neu transgenic mice. Bull Exp Biol Med 2005;139:721–723.
- (11) Memmott RM, Mercado JR, Maier CR, Kawabata S, Fox SD, Dennis PA. Metformin prevents tobacco carcinogen-induced lung tumorigenesis. Cancer Prev Res 2010;3:1066-1076.
- (12) Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JMM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 2009;32(9):1620-1625.
- (13) Currie CJ, Poole CD, Gale EAM. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009;52(9):1766-1777.
- (14) Lamanna C, Monami M, Marchionni N, Mannucci E. Effect of metformin on cardiovascular events and mortality: a meta-analysis of randomized clinical trials. Diabetes Obes Metabol 2011;13(3):221-228.
- (15) Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009;60:392–395.
- (16) Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Scharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci 2011;66:191–201.
- (17) Coppé J-P, Desprez P-Y, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Ann Rev Pathol 2010;5:99-118.
- (18) Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol 1956;11(3):298–300.
- (19) Lane N. A unifying view of ageing and disease: the double-agent theory. J Theor Biol 2003;225:531-540.
- (20) Stephenne X, Foretz M, Taleux N, van der Zon GC, Sokal E, Hue L, Viollet B, Guigas B. Metformin activates AMP-activated protein kinase in primary human hepatocytes by decreasing cellular energy status. Diabetologia 2011;54(12):3101-3110.
- (21) Larsen S, Rabøl R, Hansen CN, Madsbad S, Helge JW, Dela F. Metformin-treated patients with type 2 diabetes have normal mitochondrial complex I respiration. Diabetologia 2011; [Epub ahead of print].
- (22) Saleter S, Greyber E, Pasternak G, Salpeter E. Risk of fatal and non-fatal lactic acidosis with metformin in type 2 diabetes. Archives Int Med 2003;163:2294-2602.
- (23) Roche C, Nau A, Peytel E, Moalic JL, Oliver M. Acidose lactique schevere par intoxication accidentele à la metformine: à propos de 3 observations. Ann Biol Clin (Paris) 2011;69(6):705-711.
- (24) Misbin RI, Green L, Stadel BV, Gueriguian JL, Gubbi A, Fleming GA. Lactic acidosis in patients with diabetes treated with metformin. New Engl J Med 1998;338:265-266.
- (25) http://www.longecity…/crosslinks-r20
- (26) Ishibashi Y, Matsui T, Takeuchi M, Yamagishi SI. Beneficial effects of metformin and irbesartan on advanced glycation end products (AGEs)-RAGE-induced proximal tubular cell injury. Pharmacol Res 2011;[Epub ahead of print].
- (27) Flurkey K, Papaconstantinou J, Harrison DE. The Snell dwarf mutationPit1dw can increase life span in mice. Mech Ageing Dev 2002;123(2-3):121-130.
- (28) Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature 1996;384:33.
- (29) Barbieri M, Bonafè M, Franceschi C, Paolisso G. Insulin/IGF-1-signaling pathway: an evolutionary concerved mechanism of longevity from yeast to humans. Am J Physiol Endocrinol Metab 2003;285(5):E1064-1071.
- (30) Halicka HD, Zhao H, Li J, Traganos F, Zhang S, Lee M, Darzynkiewicz Z. Genome protective effect of metformin as revealed by reduced level of constitutive DNA damage signaling. Aging (Albany NY) 2011;3(10):1028-1038.
- (31) de Jager J, Kooy A, Lehert P, Wulffelé MG, van der Kolk J, Bets D, Verburg J, Donker AJM, Stehouwer CDA. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B12 deficiency: randomized placebo controlled trial. BMJ 2010;3401:c2181.
- (32) Bauman WA, Shaw S, Jayatilleke E, Spungen AM, Herbert V. Increased intake of calcium reverses vitamin B12 malabsorption induced by metformin. Diabetes Care 2000;23:1227-1231.
Metformin a life extension drug? – Articles – Articles – portal – LONGECITY.